Our Science.
We are targeting dysregulated RNA polymerase I (Pol I) transcription in MYC-driven cancers. Pol I transcription of ribosomal RNA genes (rDNA) is tightly regulated downstream of oncogenic pathways, and its dysregulation is a common feature in cancer and other human diseases.
MYC serves as the main regulator of RNA Pol I transcription and controls Pol I transcription directly by binding to the ribosomal RNA gene (rDNA) repeats and indirectly by facilitating RNA Pol II mediated transcriptional activation of a large cohort of Pol I associated transcription factors. MYC-downstream effect is up-regulation of Pol I transcription, resulting in increased synthesis of rRNA, ribosome biogenesis, and cell growth. Because the exclusive function of Pol I is the transcription of rRNA, which is the rate limiting step of ribosomal biogenesis, the inhibition of Pol I transcription presents a novel approach for therapeutic intervention using a biomarker-driven precision medicine strategy.
Pol I Transcription is a Critical
Component in MYC-driven Cancers
MYC overexpression is seen in a high percentage of many types of cancers, including ovarian, esophageal, breast, prostate, head and neck, liver and more. Overexpression of MYC in cancer cells causes increases of both rRNA and ribosomal protein (RP) allowing for an increased number of ribosomes necessary to maintain uncontrolled growth and proliferation.
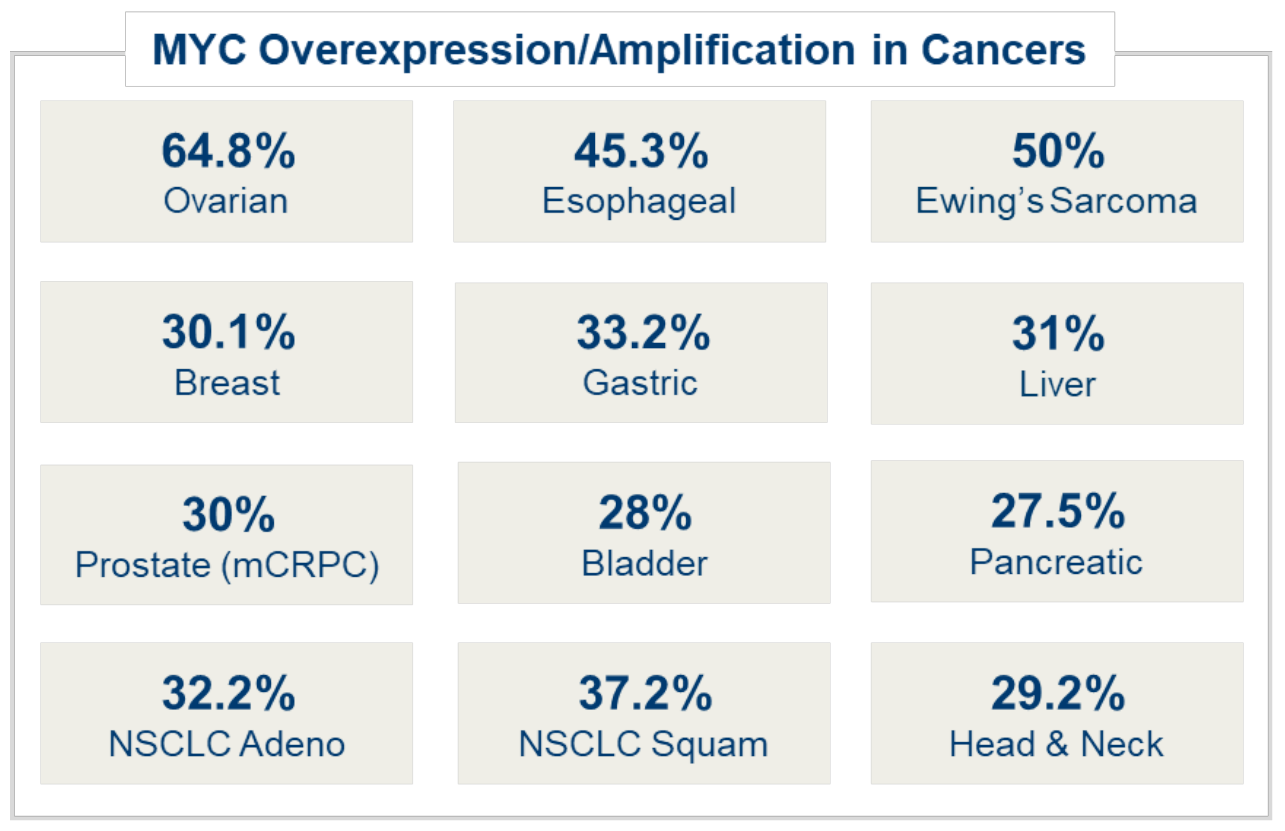
Company information, compiled industry data and Pediatric and Developmental Pathology 2017, Vol. 20(3) 213–223 2017, Society for Pediatric Pathology
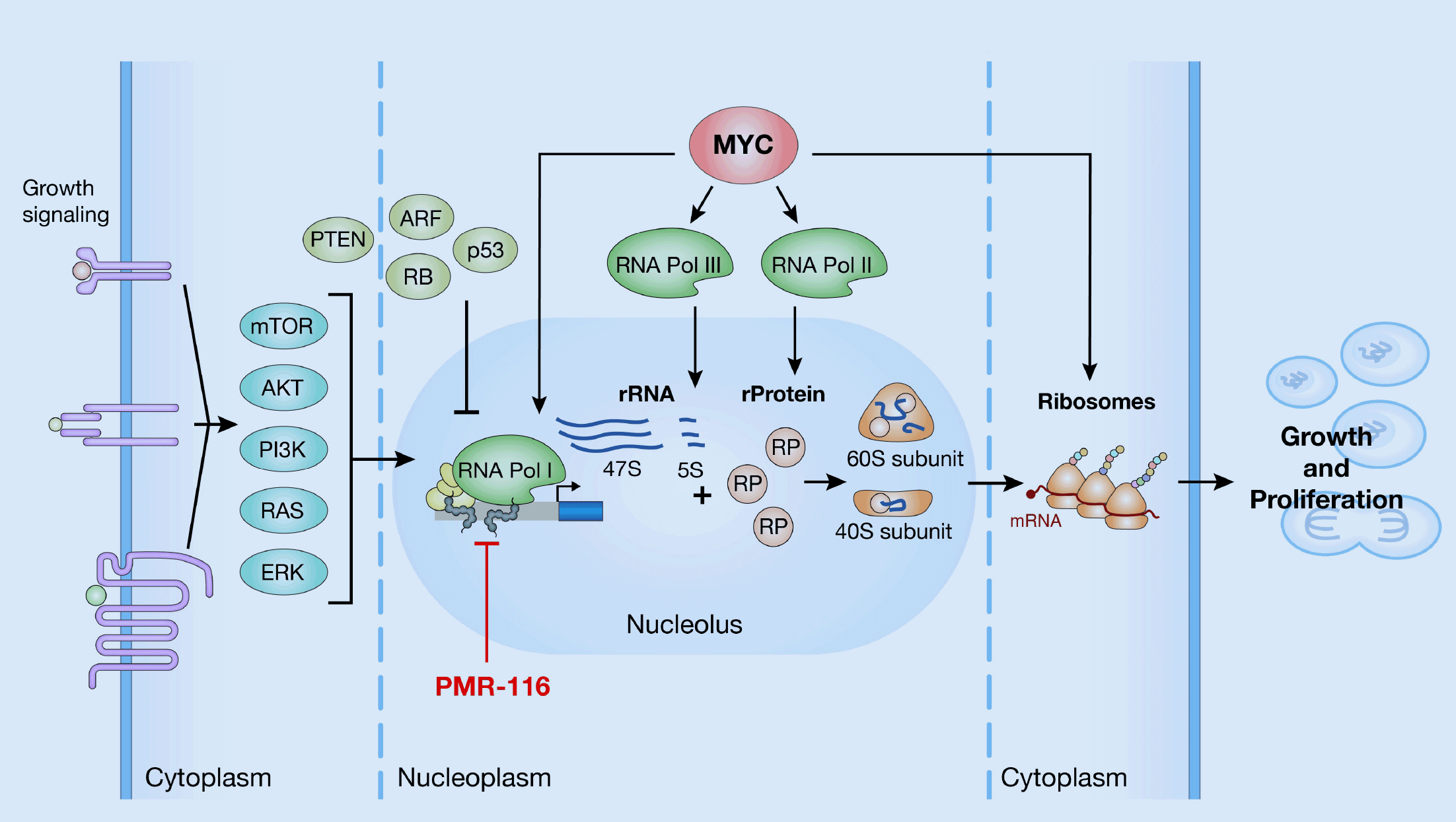
We have designed our lead program to inhibit Pol I transcription, resulting in reduction of rRNA levels, and therefore, excess free RP activates several pathways that promote growth inhibition or cancer cell death. We believe that screening for patients with high overexpression of MYC in clinical studies can help increase response to PMR-116.
Our Pipeline

About PMR-116
We have demonstrated robust preclinical efficacy in multiple MYC-driven models with PMR-116, including those that are resistant to standard-of-care treatments.
PMR-116 is currently in the dose escalation stage of a Phase 1a/b clinical trial. We intend to expand the development of PMR-116 in patients with MYC overexpressing solid tumors in a tumor type-agnostic approach.
For more information about our ongoing clinical trial, please visit ANZCTR.
